

2013 , Vol. 07 >Issue 04: 238 - 244
DOI: https://doi.org/10.3877/cma. j. issn.1674-0807.2013.04.001
Wilms 瘤基因1 低表达和过表达乳腺癌细胞模型的建立
Copy editor: 罗承丽
收稿日期: 2013-07-10
网络出版日期: 2024-12-05
基金资助
国家自然科学基金资助项目(81102030)
版权
Establishment of the Wilms’ tumor gene 1 low and over-expression models in breast cancer cells
Received date: 2013-07-10
Online published: 2024-12-05
Copyright
目的
应用RNA 干扰(RNAi)和RNA 激活(RNAa)技术,分别构建小干扰RNA(siRNA)和双链RNA(dsRNA),建立Wilms 瘤基因1(WT1)低表达和过表达的乳腺癌细胞模型。
方法
以国外学者提供的3 条序列(siRNA-516、siRNA-803、siRNA-1029)构建WT1 siRNA 干扰表达WT1,以研究已证实可上调WT1 表达的序列(dsRNA-319)构建dsRNA 过表达WT1,通过脂质体(LipofectamineTM 2000)分别将siRNA 和dsRNA 转入MDA-MB-321 和MCF-7 细胞。 WT1 有效siRNA 筛选实验分为6 组:WT1 siRNA-516、WT1 siRNA-803、WT1 siRNA-1029、阴性对照、脂质体组和空白细胞组;观察转染效率的时间点为转染后24、48、72 h。 WT1 dsRNA 的筛选实验分为3 组:WT1 dsRNA-319、阴性对照组和空白细胞组;观察转染效率的时间点为转染后48、72、96 h。 通过实时定量PCR(qRT-PCR)和Western Blotting 筛选作用效果最明显的siRNA 和dsRNA。 使用单因素方差分析进行统计学分析。
结果
成功构建WT1 siRNA-516、WT1 siRNA-803 和WT1 siRNA-1029 共3 个siRNA,并转染到MDA-MB-231 细胞中,转染效率达90%以上。 上述3 个WT1 siRNA 均能够抑制WT1 mRNA 和蛋白的表达,以转染后48 h WT1 siRNA-1029的效果最为显著[WT1 siRNA-1029 组WT1 mRNA 表达水平与空白细胞组相比显著降低(0.49±0.02 比1.00±0.08,P=0.00),其蛋白表达水平也明显降低]。 成功将WT1 dsRNA-319 转染到MCF-7 细胞中,转染效率达90%以上。 50 μmol/L 的WT1 dsRNA-319 转染后96 h,MCF-7 细胞的WT1 过表达最为显著[WT1 dsRNA-319 组的WT1 mRNA 表达水平与空白细胞组相比显著升高(319.06±14.84 比1.00±0.07,P=0.00),其蛋白表达水平也明显升高]。
结论
成功建立低表达WT1 的MDA-MB-231 细胞和过表达WT1 的MCF-7 细胞模型,为后续进一步研究WT1 在乳腺癌中的生物学行为奠定了基础。
关键词: Wilms 瘤基因1; RNA 干扰; RNA 激活; 乳腺肿瘤
齐晓伟 , 杨新华 , 张毅 , 范林军 , 张帆 , 陈莉 , 唐振宁 , 张彦 , 杨晓宁 , 宗贝歌 , 胡保全 , 王明浩 , 姜军 . Wilms 瘤基因1 低表达和过表达乳腺癌细胞模型的建立[J]. 中华乳腺病杂志(电子版), 2013 , 07(04) : 238 -244 . DOI: 10.3877/cma. j. issn.1674-0807.2013.04.001
Objective
To construct small interfering RNA (siRNA) and double strands RNA(dsRNA) by RNA interference (RNA interference, RNAi) and RNA activation (RNA activating, RNAa),and establish the low and over-expression model of Wilms’ tumor gene 1 (WT1) in breast cancer cells.
Methods
Three sequences (siRNA-516, siRNA-803 and siRNA-1029) established by foreign scholars were adopted to construct WT1 siRNA; one sequence (dsRNA-319)which demonstrated to be able to up-regulate WT1 expression was adopted to construct WT1 dsRNA. siRNA and dsRNA were transfected into MDA-MB-321 and MCF-7 cells by LipofectamineTM 2000,respectively. WT1 siRNA screening experiment contained six groups:WT1 siRNA-516, WT1 siRNA-803, WT1 siRNA-1029, negative control, transfection reagent and blank groups, and the points of time for observation were at 24, 48 and 72 h after transfection, respectively. WT1 dsRNA screening experiments contained three groups: WT1 dsRNA-319, negative control and blank group, and the point of time for observation was at 48, 72 and 96 h after transfection respectively. Quantitative real-time PCR(qRT-PCR)and Western Blotting were performed to select siRNA and dsRNA with obvious impacts on WT1 expression. The one-way ANOVA was used for statistical analysis.
Results
Three WT1 siRNAs (WT1 siRNA-516, WT1 siRNA-803 and WT1 siRNA-1029) were successfully constructed and transfected into MDAMB-231 cells with transfection efficiency >90%. WT1 siRNAs could effectively inhibit the expression of WT1 mRNA and protein, among which siRNA-1029 could inhibit the WT1 mRNA expression at 48 h after transfection most significantly (0.49±0.02 for WT1 siRNA-1029 group vs 1.00±0.08 for blank group,P=0.00;the protein expression was also decreased dramatically). WT1 dsRNA-319 could increase the expression of WT1 mRNA in MCF-7 cell with transfection efficiency >90%. The most significant impact was achieved in 50 μmol/L WT1 dsRNA-319 group at 96 h after transfection (319.06±14.84 for WT1 dsRNA-319 group vs 1.00±0.07 for blank group, P=0.00; the protein expression was also increased remarkably).
Conclusion
The low expression WT1 model in MDA-MB-231 cells and the over-expression WT1 model in MCF-7 cells are successfully established, which provides a basis for subsequent study on WT1 biological behaviors in breast cancer.
Key words: Wilms’ tumor gene 1; RNA interference; RNA activating; Breast neoplasms
表1 Wilms 瘤基因1(WT1) 小干扰RNA(siRNA)序列 |
| siRNA | 靶点 | 序列 | 荧光标记 |
|---|---|---|---|
| WT1 siRNA-516 | 外显子4 | 5′-GACAAUUUAUACCAAAUGATT-3′ | FAM |
| WT1 siRNA-803 | 外显子7 | 5′-GCUGUCCCACUUACAGAUGdTdT-3′ | FAM |
| WT1 siRNA-1029 | 外显子10 | 5′-AAGCCCUUCAGCUGUCGGUdTdT-3′ | FAM |
| WT1 siRNA-NC | 5′-CGUACGCGGAAUACUUCGAdTdT-3′ | FAM |
表2 Wilms 瘤基因1(WT1) 双链RNA(dsRNA)序列 |
| dsRNA | 序列 | 末端标记 |
|---|---|---|
| WT1 dsRNA-319 | 5′-FGACUCACUGCUUACCUGAA[dT][dT] -3′ | HPLC |
| 5′-RUUCAGGUAAGCAGUGAGUC[dT][dT] -3′ | ||
| WT1 dsRNA-NC | 5′-ACUACUGAG UGACAGUAGA[dT][dT] -3′ | FAM |
| 5′-UCUACUGUCACUCAGUAGU[dT][dT] -3′ |
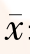 ±s 表示,多组数据比较采用单因素方差分析,组间两两比较采用LSD(方差整齐) 或者Tamhane’s T2(M)(方差不整齐)检验,以P<0.05为差异有统计学意义。
±s 表示,多组数据比较采用单因素方差分析,组间两两比较采用LSD(方差整齐) 或者Tamhane’s T2(M)(方差不整齐)检验,以P<0.05为差异有统计学意义。图1 显微镜下观察不同比例的脂质体与羧基荧光素(FAM)-小干扰RNA 转染MDA-MB-231 细胞的效率(×200)a,e:0.25 μl 脂质体∶2.5 pmol siRNA; b,f:0.25 μl 脂质体∶5 pmol siRNA; c,g:0.5 μl 脂质体与∶5 pmol siRNA; d,h:1 μl 脂质体∶10 pmol siRNA (a ~d:倒置相差显微镜观察;e ~h:荧光显微镜观察) |
图3 Western Bloting 检测WT1 小干扰RNA 转染后24、48、72 h MDA-MB-231 细胞中WT1 蛋白的表达水平a:转染后24 h;b:转染后48 h;c:转染后72 h 1 ~6 分别代表WT1 siRNA-516、WT1 siRNA-803、WT1 siRNA-1029、阴性对照、脂质体和空白细胞组; WT1: Wilms 瘤基因1 |
表3 siRNA 转染后24、48、72 h 各组MDA-MB-231细胞的WT1 mRNA 相对表达量比较 |
| 组别 | 实验次数 | WT1 mRNA 相对表达量 | ||
|---|---|---|---|---|
| 24 ha | 48 hb | 72 hb | ||
| 空白细胞组 | 3 | 1.00±0.08 | 1.00±0.08 | 1.00±0.01 |
| 脂质体组 | 3 | 0.94±0.04 | 0.94±0.04 | 0.85±0.02 |
| 阴性对照组 | 3 | 1.02±0.18 | 0.91±0.09 | 0.93±0.02 |
| WT1 siRNA-516组 | 3 | 0.75±0.05 | 0.49±0.03c | 0.55±0.02c |
| WT1 siRNA-803组 | 3 | 0.66±0.05 | 0.45±0.04c | 0.58±0.01c |
| WT1 siRNA-1029组 | 3 | 0.71±0.06 | 0.49±0.02c | 0.70±0.01c |
| F值 | 9.60 | 63.35 | 345.71 | |
| P值 | 0.00 | 0.00 | 0.00 | |
图5 Western Bloting 检测WT1 dsRNA 转染后96 h MCF-7细胞的WT1 蛋白表达水平Blank:空白细胞组;WT1:Wilms 瘤基因1;dsRNA:双链RNA |
表4 dsRNA 转染48、72、96 h 后各组MCF-7 细胞的WT1 mRNA 相对表达量比较 |
| 组别 | 实验次数 | mRNA 相对表达量 | ||
|---|---|---|---|---|
| 48 ha | 72 hb | 96 hb | ||
| 空白细胞组 | 3 | 1.00±0.07 | 1.00±0.08 | 1.00±0.07 |
| 阴性对照组 | 3 | 0.94±0.02 | 0.92±0.05 | 0.94±0.03 |
| WT1 dsRNA-319 | 3 | 0.69±0.07c | 1.53±0.01c | 319.06±14.84c |
| F值 | 22.28 | 117.58 | 1378.27 | |
| P值 | 0.00 | 0.00 | 0.00 | |
| [1] |
Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin,2011,61(2) :69-90.
|
| [2] |
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation[J]. Cell,2011,144(5):646-674.
|
| [3] |
Huff V. Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene [J]. Nat Rev Cancer, 2011,11(2):111-121.
|
| [4] |
齐晓伟, 姜军. Wilms 瘤基因1 与实体肿瘤发生、演进关系的研究进展[J]. 中华外科杂志,2013,51(5):461-463.
|
| [5] |
Qi XW, Zhang F, Yang XH, et al. High Wilms’ tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer [J].Oncol Rep,2012,28(4):1231-1236.
|
| [6] |
Miyoshi Y, Ando A, Egawa C, et al. High expression of Wilms’ tumor suppressor gene predicts poor prognosis in breast cancer patients [J]. Clin Cancer Res,2002,8(5):1167-1171.
|
| [7] |
Huang V,Qin Y,Wang J,et al. RNAa is conserved in mammalian cells [J]. PLoS ONE,2010,5(1):e8848.
|
| [8] |
Qin Q, Lin YW, Zheng XY, et al. RNAa-mediated overexpression of WT1 induces apoptosis in HepG2 cells [J].World J Surg Oncol,2012,10:11.
|
| [9] |
Perugorria MJ, Castillo J, Latasa MU, et al. Wilms’ tumor 1 gene expression in hepatocellular carcinoma promotes cell dedifferentiation and resistance to chemotherapy [J]. Cancer Res,2009,69(4):1358-1367.
|
| [10] |
刘安定, 董学君, 杨明峰. siRNA 沉默hTERT 基因表达对人乳腺癌细胞增殖和凋亡的影响[J/CD]. 中华乳腺病杂志:电子版,2008,2(5):538-546.
|
| [11] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method[J]. Methods,2001,25(4):402-408.
|
| [12] |
Yang L, Han Y, Suarez Saiz F, et al. A tumor suppressor and oncogene:the WT1 story[J]. Leukemia,2007,21(5):868-876.
|
| [13] |
Hartkamp J, Carpenter B, Roberts SG. The Wilms’ tumor suppressor protein WT1 is processed by the serine protease HtrA2/Omi [J]. Mol Cell,2010,37(2):159-171.
|
| [14] |
Vicent S, Chen R, Sayles LC, et al. Wilms tumor 1 (WT1)regulates KRAS-driven oncogenesis and senescence in mouse and human models [J]. J Clin Invest,2010,120 (11):3940-3952.
|
| [15] |
齐晓伟, 姜军. 2012 年第4 版《WHO 乳腺肿瘤组织学分类》介绍[J/CD]. 中华乳腺病杂志:电子版, 2012, 6(5):586-591.
|
| [16] |
胡小辉,李建文. RNA 干扰技术在乳腺癌治疗中的研究进展[J/CD]. 中华乳腺病杂志:电子版,2013,7(1):47-51.
|
| [17] |
Portnoy V, Huang V, Place RF, et al. Small RNA and transcriptional upregulation [J]. Wiley Interdiscip Rev RNA,2011,2(5):748-760.
|
| [18] |
Pushparaj PN, Aarthi JJ, Kumar SD, et al. RNAi and RNAathe yin and yang of RNAome[J]. Bioinformation,2008,2(6):235-237.
|
| [19] |
Place RF, Noonan EJ, Földes-Papp Z, et al. Defining features and exploring chemical modifications to manipulate RNAa activity [J]. Curr Pharm Biotechnol,2010,11(5):518-526.
|
| [20] |
Wang J, Li LC. Small RNA and its application in andrology and urology [J]. Transl Androl Urol,2012,1(1):33-43.
|
| [21] |
齐晓伟. WT1 在乳腺癌进展中的作用及机制研究[D]. 重庆: 第三军医大学,2012.
|
/
| 〈 |
|
〉 |